Lesson 11
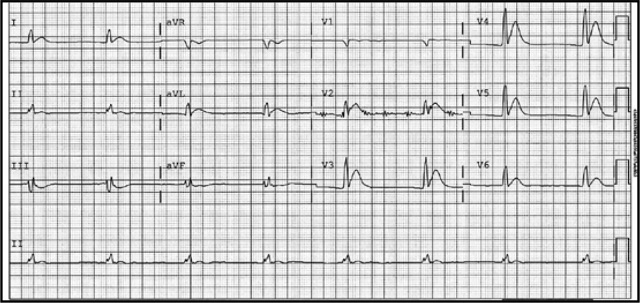
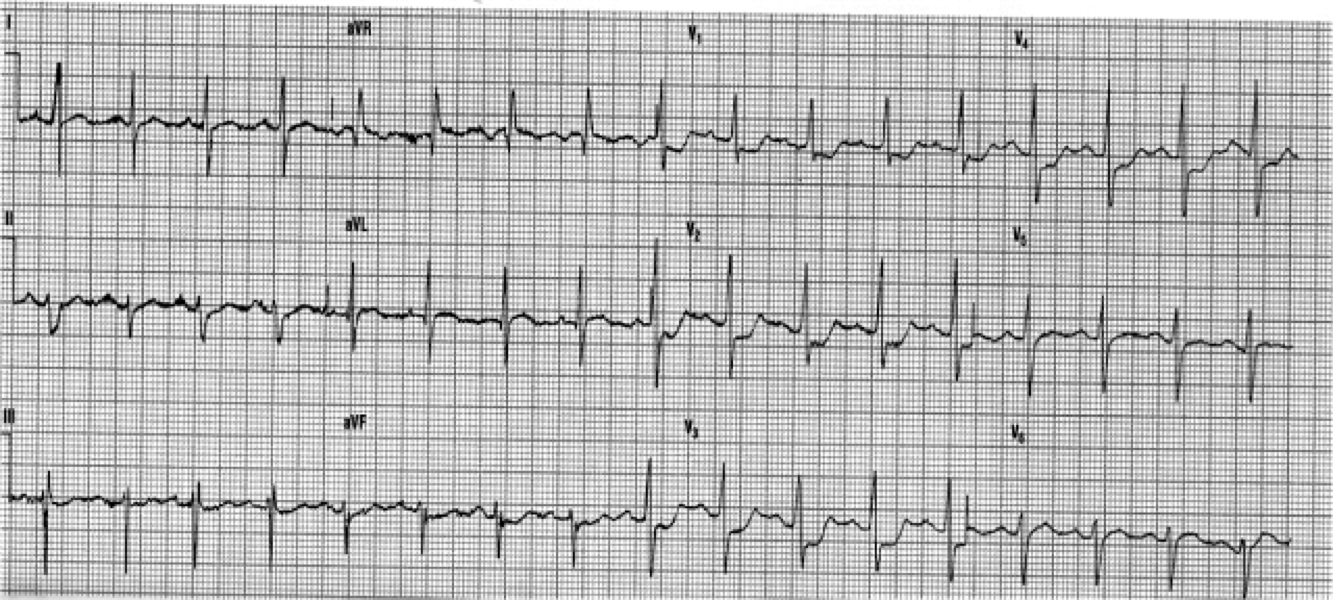
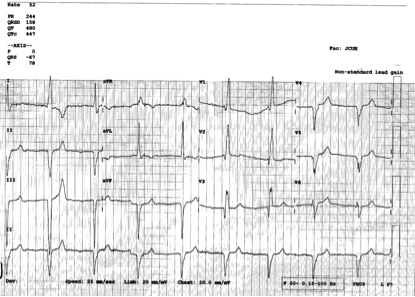
A man in his 60’s presented after 4 days of chest pain, with some increase of pain on the day of presentation. Exact pain history was difficult to ascertain. There was some SOB. He had walked into the A&E (did not use the ambulance). He was in no distress and vital signs were normal. Here is his ECG:
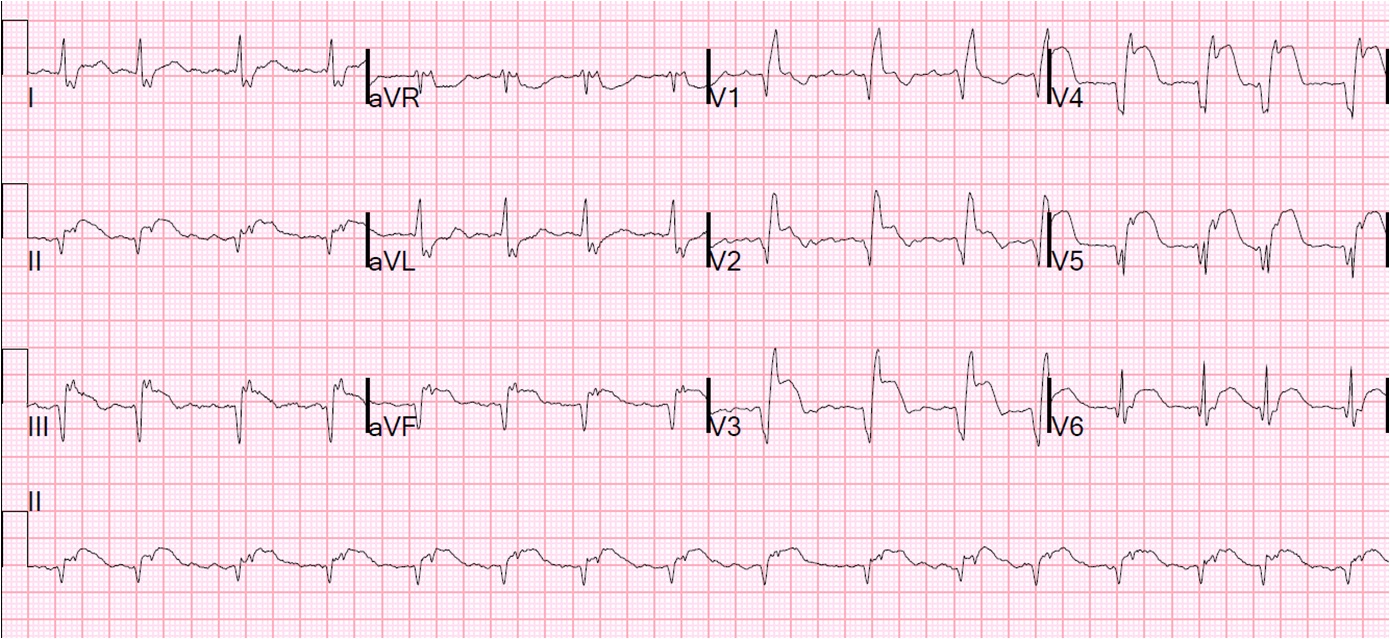
What is your interpretation? Obviously there is MI. How acute is it?
Description
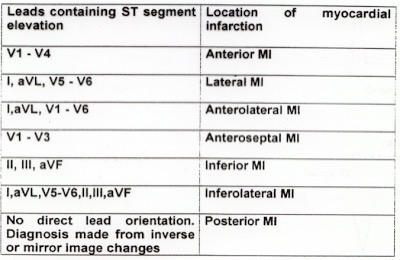
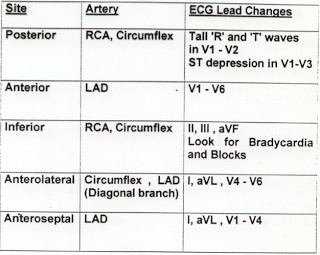
There is atrial fibrillation at a rate of 95. There is Right Bundle Branch Block with a QR particularly noted in V1-V3 (no rSR’, because there is an initial Q-wave; this is diagnostic of infarction in the anterior wall and septum). The Q-waves extend to V5 and are very wide (80 ms in V2). There are also inferior Q-waves which can mimic a left anterior fascicular block, as they result in left axis deviation. There is rather massive ST elevation, and this is not only anterior but inferior (see analysis below).
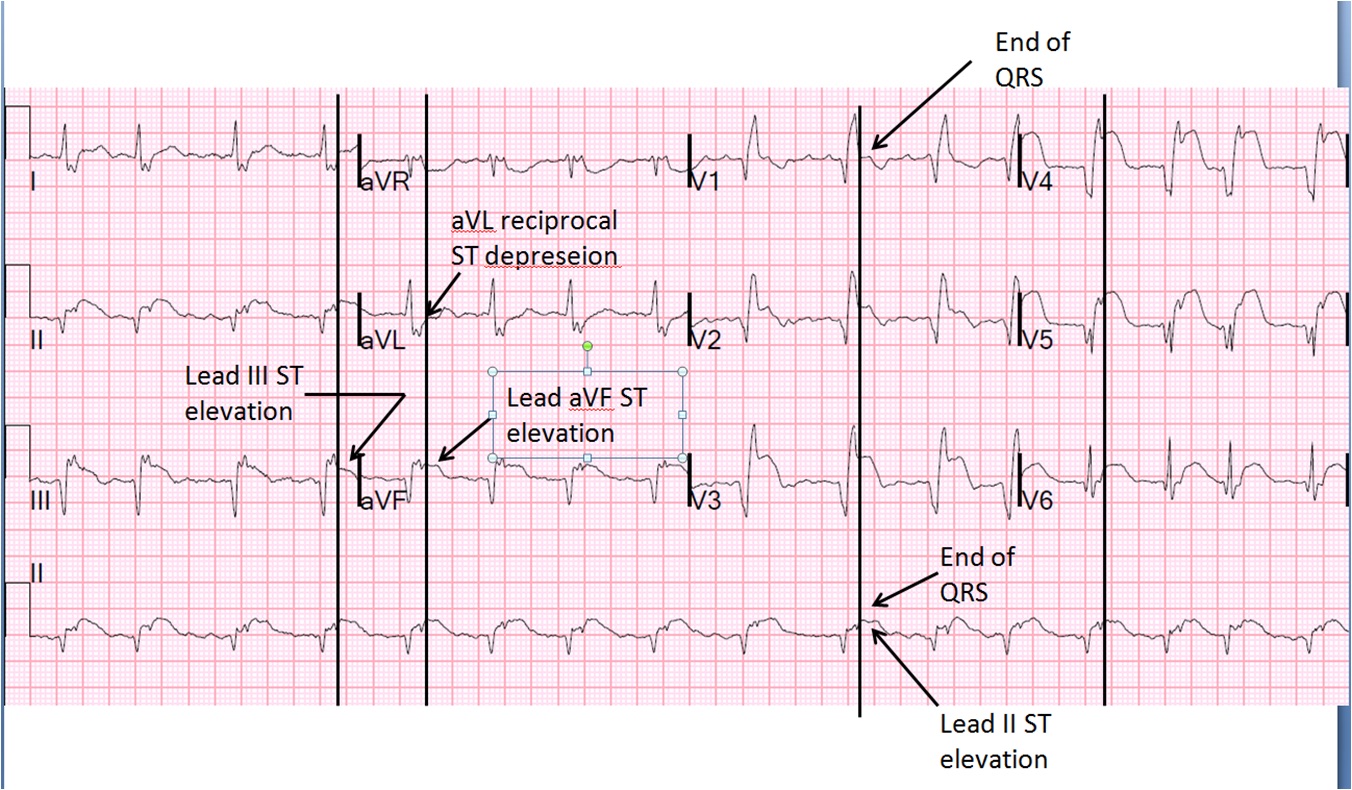
The end of the QRS is best seen in lead V1 (and results in a QRS duration of 176 ms). If one draws a line down to lead II across the bottom, one can find the end of the QRS in lead II. From there, one can find the end of the QRS in all leads. This analysis shows that there is ST elevation after the end of the QRS in lead II, III, and aVF, and reciprocal ST depression in aVL. Thus, this is BOTH an anterior and inferior STEMI in the setting of RBBB.
How old is this antero-inferior STEMI?
Could it be acute (vs. subacute or days old)? Although the patient has had pain for 4 days, could the artery have fully occluded only within hours? Very unlikely. Although acute anterior STEMI frequently has narrow QR-waves within one hour of onset (1. Raitt et al.), such Q-waves are associated with larger MI and worse outcomes (2. Armstrong et al.), the presence of such well developed, wide, anterior Q-wave suggests completed transmural STEMI.
So this patient likely has a several day old infarction, with persistent ST elevation and persistently upright T-waves.
The wide Q-waves suggest “transmural” MI (completed MI with infarction of the entire thickness of the ventricle). This was common in the days before reperfusion of STEMI, but still happens in patients who present late and therefore do not get timely reperfusion therapy. When there is MI extending all the way to the epicardium (transmural), that infarcted epicardium is often inflamed (postinfarction regional pericarditis, or PIRP).
What complication is the patient with post-infarction regional pericarditis at risk for?
The patient was taken to the cath lab emergently and a 100% mid LAD occlusion was opened but opening resulted in no flow. The initial troponin I was 23.7 ng/ml and was falling, confirming infarction days ago.
Case Continued
2 days later the patient became increasingly tachycardic, hypotensive, clammy (in cardiogenic shock) and had a new murmur. This was the 12-lead ECG.
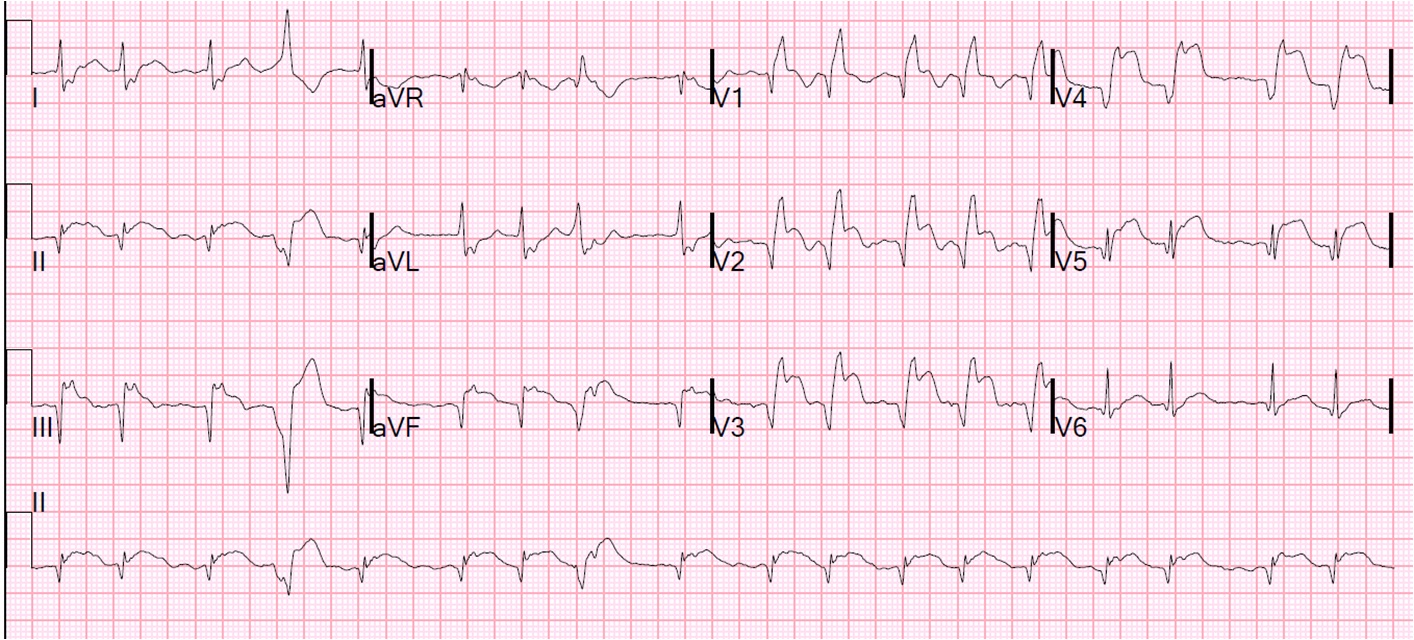
Not much change, except a slightly faster ventricular response at 110bpm. No resolution of ST elevation. The T-waves are persistently positive. This remains consistent with PIRP, as was the first ECG. Notice also how easy it is to diagnose ST elevation in the PVC.
An echocardiogram showed no hemopericardium, but Doppler showed a new small ventricular septal defect with left to right shunting. This was in addition to a large septal, anterior, and apical wall motion abnormality, and moderately severely decreased LV function.
An intra-aortic balloon pump was placed, and the patient was taken for immediate surgical repair but did not survive.
Discussion
When there is full thickness infarction, there is epicardial inflammation (post-infarction regional pericarditis), and the myocardium is at risk of “rupture.” The term “rupture” makes it sound like some sort of explosion or massive blowout, but it is usually a small, slow leak that, over time, can cause tamponade and death. Rupture can be either free wall rupture (causing tamonade) or septal rupture, causing ventricular septal defect with left to right flow and resulting pulmonary edema and shock. If detected early by ultrasound, the patient can be saved. Our own Dave Plummer of HCMC reported on survival of 2 of 6 patients with STEMI who had free wall myocardial rupture diagnosed by presence of hemopericardium on bedside ultrasound in the ED.(3)
Oliva et al. (4) strongly associated myocardial rupture with postinfarction regional pericarditis (PIRP), and associated PIRP with persistent upright T-waves. He found 2 ECG patterns of atypical T-wave development in PIRP:
1) persistently positive (upright) T-waves 48 hours after AMI onset.
2) premature, gradual reversal of inverted T waves to positive (upright) deflections by 48 to 72 hours after MI onset in the presence of well formed Q-waves.
Lessons:
1. Well formed Q-waves with persistent ST elevation, especially in a patient with prolonged pain, should alert to transmural MI with possible post-infarction pericarditis. One should be on the alert for myocardial rupture.
2. In the case of septal MI, as here, be on the alert for development of a ventricular septal defect.
3. Bedside echo may detect these in a timely way.
4. Additionally, these patients have a high incidence of LV aneurysm with mural thrombus.
5. Although rupture has a high mortality, it is not uniformly fatal
References
1. Raitt MH, Maynard C, Wagner GS, Cerqueira MD, Selvester RH, Weaver WD. Appearance of abnormal Q waves early in the course of acute myocardial infarction: implications for efficacy of thrombolytic therapy. J Am Coll Cardiol 1995;25(5):1084-8.
2. Armstrong PW et al. Baseline Q-wave surpasses time from symptom onset as a prognostic marker in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. J Am Coll Cardiol 53(17):1503-9. Apr 28, 2009.
3. Plummer D. Dick C. Ruiz E. Clinton J. Brunette D. Emergency department two-dimensional echocardiography in the diagnosis of nontraumatic cardiac rupture. Ann Emerg Med 1994; 23(6):1333-42.
4. Oliva PB, Hammill SC, Edwards WD. Electrocardiographic diagnosis of postinfarction regional pericarditis: ancillary observations regarding the effect of reperfusion on the rapidity and amplitude of T wave inversion after acute myocardial infarction. Circulation 1993;88(3):896-904.
5. Oliva PB. Hammill SC. Edwards WD. Cardiac rupture: a clinically predictable complication of acute myocardial infarction: report of 70 cases with clinicopathologic correlations. J Am Coll Cardiol 1993;22(3):720-6